Image Source: “Smoking has a Lasting Impact on the Immune System, 2024”
When we get sick, our bodies’ immune systems work to fight off infections by invading pathogens, or organisms like bacteria and viruses that cause disease. However, many factors such as lack of sleep and poor nutrition weaken our immune system, meaning that we are less able to stay healthy. It has been known that smoking is another one of these factors that weaken our immune systems, but a recent study from a group at the Institut Pasteur in France looking at the effects of a variety of factors on the immune system showed that the extent to which smoking plays a role is much higher than many would think. But to understand the results of this study, it is important to first understand the mechanisms the immune system uses to fight infection.
The immune system has many different moving components, including two distinct branches. The first is the faster, more general innate immune system which has a similar response to all infections. The second is the adaptive immune system which is slower, memory-based, and is involved in pathogen specific response. Although the innate immune system involves general molecules that interact with all cells and the adaptive immune system has specialized molecules that interact with pathogens based on memory of past infection, they share one important class of signaling molecules. These molecules are called cytokines and their role is to coordinate both of these types of immune response. Cytokines are small molecules that are released by immune cells to communicate with other parts of the body and each other. This signaling results in deployment of a response by other immune cells against invading pathogens. However, levels of cytokine production exist in a very fine balance. In order to get the desired immune response, you need the exact right level of cytokines present. If levels are too high or too low, they could cause abnormalities including overactive immune response and inflammation or impaired immune responses.
To investigate the effects of a variety of different factors on the immune system and cytokine responses of healthy individuals, a project called the Milieu Intérieur put together a cohort of 1000 healthy participants and has been studying variability in the immune system between these individuals (“The Milieu Intérieur Project”). In an investigation of this data, the group from Institut Pasteur, Saint André et al, analyzed 136 variables measured in the Milieu Intérieur Project that could be causing differences in cytokine secretion and immune response (Luo and Stent 2024). These variables included everything from demographics, to diet, to health habits like smoking, to social and environmental characteristics (Saint-André et al. 2024).
After they performed their initial statistical analysis, Saint André et al measured production of 13 disease relevant cytokines as a quantitative measure of immune response in populations with different demographics, health habits, and other characteristics. In the lab, they exposed blood samples from their sample population to 12 different molecules meant to serve as stimulants for the immune system (these molecules included things like viral and bacterial proteins). After this exposure, the authors tested cytokine production in both innate and adaptive immune cells, and once they had that data, they took their results one step further. The group also used epigenetics, or the study of changes in gene expression rather than the DNA code that makes up the genome to investigate possible reasons for variability in immune responses associated with factors tested. Their epigenetic evaluation consisted of analyzing the extent to which one epigenetic process, DNA methylation, occurred at specific regulators of signaling and metabolism (Saint-André et al. 2024) to assess changes associated with smoking.
As previously stated, one of the authors’ main findings from the initial statistical analysis was that smoking had a large effect on cytokine response. In fact it had the same effect as age, sex, and genetics, three things many would consider much more directly impactful to the immune system than smoking. In their in vitro simulations, they found that smoking had a temporary effect on the ability of the innate immune system to function properly. This result is a relatively intuitive one. If you do something that is considered bad for you, it makes sense that you would get sick more easily.
However, more surprisingly, they also found that smoking leaves a lasting effect on memory based adaptive immune responses even after cessation of smoking, meaning that even after people quit smoking, their immune systems still are impacted. They found that in samples from individuals who smoked there were higher levels of cytokine expression, especially of an inflammatory cytokine called CXCL5 that is secreted in response to bacterial infection. Secretion of this cytokine is associated with the presence of an inflammatory protein called CEACAM6 in the blood. Consistent upregulation of levels of this protein has been found to have links with multiple cancers such as colon cancer (Wu et al. 2024). In Saint André et al’s epigenetic investigation of this association, they found that DNA methylation, which results in a downregulation of gene expression and in this case an increase in cytokine production, is linked to smoking’s lasting effect on the immune system (Greenberg and Bourc’his 2019). DNA methylation was decreased at many of the sites they tested which are involved in regulation of signaling genes and metabolism. Decreased DNA methylation was likely impacting levels of cytokines in response to detection of pathogens. In these populations, smoking caused lasting changes in gene expression which resulted in long term changes in addition to the expected short term effects on the immune system.
This study demonstrates that smoking can have lasting negative impacts on your health which are not limited to just lung damage. It is also associated with pro-inflammatory cancer pathways and epigenetic markers that cause increased cytokine production. This overproduction of cytokines can confuse cells and also cause increased inflammation. Over time the extra inflammation can damage tissues and lead to developments of other conditions, like the cancers previously mentioned and complications associated with overproduction of cytokines (“What are Cytokines”). These recent findings emphasize that it is important to consider the possible implications of smoking and all things that we expose ourselves to, and to keep in mind that new data is still being discovered.
Works Cited
The Milieu Intérieur Project Institut Pasteur. Luo,Y. and Stent,S. (2024) Smoking’s lasting effect on the immune system. Nature, 626, 724–725.
Saint-André,V., Charbit,B., Biton,A., Rouilly,V., Possémé,C., Bertrand,A., Rotival,M., Bergstedt,J., Patin,E., Albert,M.L., et al. (2024) Smoking changes adaptive immunity with persistent effects. Nature, 626, 827–835.
Wu,G., Wang,D., Xiong,F., Wang,Q., Liu,W., Chen,J. and Chen,Y. (2024) The emerging roles of CEACAM6 in human cancer (Review). International Journal of Oncology, 64, 1–15.
Greenberg,M.V.C. and Bourc’his,D. (2019) The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol, 20, 590–607.
What are Cytokines? Types and Function Cleveland Clinic.
Smoking has a lasting impact on the immune system, a new study finds (2024) Euronews.









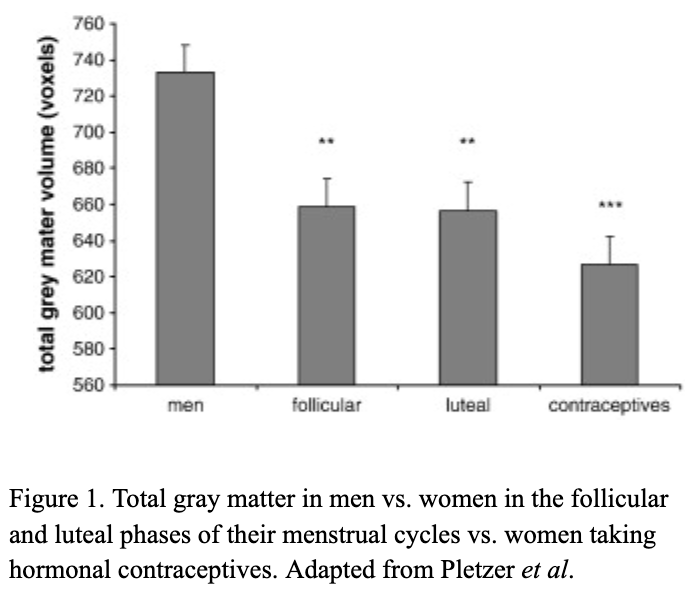
 There are millions of women taking steroids every day. But how is this possible? Are they just getting really buff? It feels like we always hear stories about how performance-enhancing drugs, namely steroids, are giving world-class athletes the boost they need to beat out their competition. But women across the globe are taking steroids every day as well, in the form of hormonal birth control. Despite their widespread use, side effects of hormonal contraceptives are largely unstudied, or have been until recently. In the last ten years, several studies have come out about the effect of taking a daily dose of steroids on women’s brains and mental health, which until now has been a severely neglected area where lack of knowledge affects millions of people worldwide.
There are millions of women taking steroids every day. But how is this possible? Are they just getting really buff? It feels like we always hear stories about how performance-enhancing drugs, namely steroids, are giving world-class athletes the boost they need to beat out their competition. But women across the globe are taking steroids every day as well, in the form of hormonal birth control. Despite their widespread use, side effects of hormonal contraceptives are largely unstudied, or have been until recently. In the last ten years, several studies have come out about the effect of taking a daily dose of steroids on women’s brains and mental health, which until now has been a severely neglected area where lack of knowledge affects millions of people worldwide. 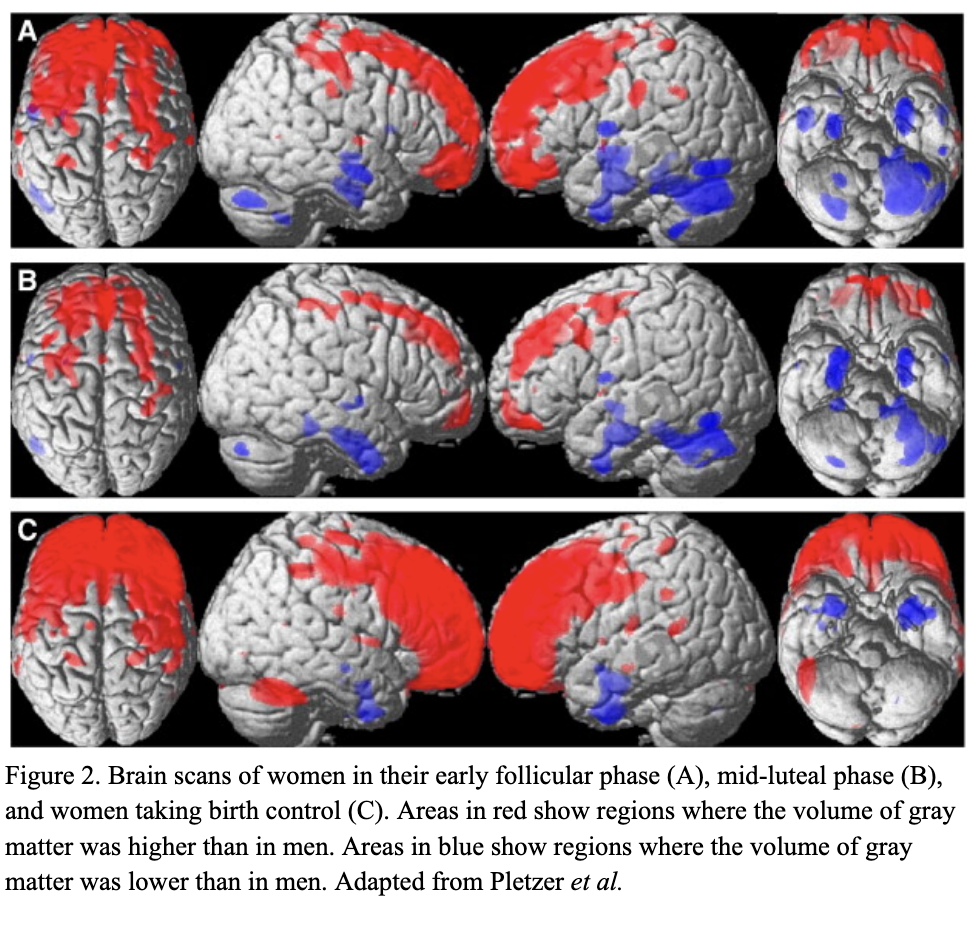 Thes
Thes