Antibiotics are medications that fight infections caused by bacteria. But they can also lead to mental health issues, such as anxiety and depression, later in life.
The discovery of antibiotics was one of the greatest medical advances of the 20th century. Antibiotics have significantly reduced mortality from infectious diseases and increased average life expectancy. However, they have a variety of side effects, including cognitive impairment and emotional disorders in adulthood (Adedji 2016).
Antibiotics destroy bacteria in the gut, which can disrupt brain function. Gut microbiota are an important component of the gut-brain axis–the two-way line of communication between the gastrointestinal tract and the central nervous system. Gut microbiota produce neurotransmitters that regulate mood, such as dopamine, norepinephrine, and serotonin, which travel through the vagus nerve to the brain (Karakan et al 2021). Previous studies have found that antibiotic-induced gut microbiota depletion causes dysfunction of the gut-brain axis, increasing anxiety and depression-related behaviors (Mosaferi et al 2021).
Aging plays an important role in the development of both gut microbiota and the brain. Microbiota first appear at birth and rapidly colonize the intestinal tract. The composition and diversity of gut microbiota resembles adult level by 2 years of age and remains stable throughout adulthood before decreasing in old age. The brain develops until the mid-to-late 20s and starts to decline in middle age. It has been shown that antibiotic-induced gut microbiota depletion has negative effects on the brain (Li et al 2022). However, no prior research has been done on this relationship during the different stages of life. This study aimed to determine the connection between gut microbiota and cognitive and emotional function during the different life stages.
In this experiment, the researchers used mice as models for human subjects, randomly assigning 75 mice to five groups. One group served as the control (Veh group) and was given distilled water from birth to death. The other four groups were given an antibiotic cocktail at different life stages: birth to death (Abx group), birth to postnatal day 21 (Abx infant group), postnatal day 21 to 56 (Abx adolescence group), and postnatal day 57 to 84 (Abx adulthood group).
At postnatal day 85, the researchers randomly selected thirteen mice from each group for testing. They measured the cognitive function and emotion of the mice by using four traditional behavioral tests: open-field test (OFT), passive avoidance test (PAT), morris water maze test (MWM), and tail suspension test (TST). The OFT measured anxiety level by observing how long the mice moved in an open field for 5 minutes. The PAT measured short-term memory, which was defined as the difference in latency–the time it took mice to reenter a room that delivered an electric shock–between day 1 and day 2 (Jahn-Eimermacher et al 2011). The MWM measured spatial memory, which was defined as incubation time, or how long it took mice to find a submerged platform in a pool after a 5-day training period. The TST measured depression state by observing the duration of quiescence (motionless state) of the mice while they were suspended upside down for 4 minutes.




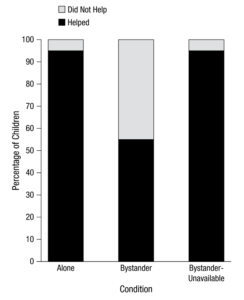
Figure 1 | Results of OFT, PAT, MVM, and TST tests. Researchers conducted four traditional behavioral tests on mice given water, as well as mice given antibiotics at different stages of life: birth to death, infancy, adolescence, and adulthood. They found that exposure to antibiotics from birth to death and in infancy led to the most severe cognitive and emotional dysfunction, followed by exposure in adolescence and adulthood (Li et al 2022).
The results of this study suggested that life cycle stages influence the relationship between gut microbiota and cognitive and emotional function. For the OFT test, the total movement time of the Abx, Abx infant, and Abx adolescent groups was significantly lower than the Veh group, indicating they were more anxious than the Veh group (Figure 1). In other words, exposure to antibiotics from birth to death, in infancy, and in adolescence caused anxiety-related behaviors. For the PAT test, the difference in latency for every Abx group was significantly lower than the Veh group, meaning all Abx groups reentered the room with the electric shock more quickly after training. These results signaled that short-term memory loss was greater in the Abx groups than the Veh group; exposure to antibiotics at any stage of life caused short-term memory loss. The MWM test found that the incubation time after the 5-day training period was significantly higher for the Abx and Abx infant groups than the Veh group, so they experienced more spatial memory loss than the Veh group; exposure to antibiotics from birth to death and in infancy caused spatial memory loss. The TST test found that the duration of quiescence in the Abx and Abx infant groups was significantly higher than the Veh group, implying they were more depressed than the Veh group. In other words, exposure to antibiotics from birth to death and in infancy caused depression-related behaviors.
The researchers’ findings align with previous work showing that depletion of gut microbiota causes cognitive impairment and emotional problems (Lach et al 2020). Furthermore, the researchers demonstrated that life cycle stages are an important factor in the relationship between gut microbiota and cognitive and emotional function. In particular, their findings strengthened the idea that infancy is a crucial stage of development of gut microbiota and the brain (Hunter et al 2023). Gut microbiota lost in infancy recovers over time; however, this depletion has lasting cognitive effects. In this study, mice given antibiotics in infancy exhibited similar behaviors in adulthood as mice given antibiotics from birth to death: anxiety, depression, memory loss, and learning ability decline. Exposure to antibiotics in infancy and in the long term led to the most severe cognitive and emotional dysfunction, followed by exposure in adolescence and adulthood.
The researchers’ findings also have implications for the treatment of mental health illnesses. Previous studies have shown that probiotics replace depleted gut microbiota, alleviating symptoms of anxiety and depression. Antidepressants and anxiolytics–current medications for anxiety and depression–cause side effects such as nausea, weight gain, insomnia, constipation, dizziness, agitation, and restlessness. Probiotics are associated with milder side effects, including gas and bloating (Bistas et al 2023). Probiotics are unlikely to treat severe depression and anxiety, but they are promising treatments for people with milder conditions. The next step is to identify and manufacture effective probiotics, which would revolutionize the field of psychiatry and improve the lives of people around the world.
References
Adedji, W.A. 2016. THE TREASURE CALLED ANTIBIOTICS. Annals of Ibadan Postgraduate Medicine. 14(2):56-57. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5354621/.
Bistas KG, Tabet JP. 2023. The Benefits of Prebiotics and Probiotics on Mental Health. Cureus Journal of Medical Science. 15(8):e43217. doi:10.7759/cureus.43217.
Hunter S, Flaten E, Petersen C, Gervain J, Werker JF, Trainor LJ, Finlay BB. 2023. Babies, bugs and brains: How the early microbiome associates with infant brain and behavior development. PLOS One. 18(8):e0288689. doi:10.1371/journal.pone.0288689.
Jahn-Eimermacher A, Lasarzik I, Raber J. 2011. Statistical analysis of latency outcomes in behavioral experiments. Behavioural Brain Research. 221(1):271-275. doi:10.1016/j.bbr.2011.03.007.
Karakan T, Ozkul C, Akkol EK, Bilici S, Sobarzo-Sánchez E, Capasso R. 2021. Gut-Brain Microbiota Axis: Antibiotics and Functional Gastrointestinal Disorders. Nutrients. 13(2):389. doi:10.3390/nu13020389.
Lach G, Fülling C, Bastiaanssen TFS, Fouhy F, O’Donovan AN, Ventura-Silva AP, Stanton C, Dinan TG, Cryan JF. 2020. Translational Psychiatry. 10(1):382. doi:10.1038/s41398-020-01073-0.
Li J, Pu F, Peng C, Wang Y, Zhang Y, Wu S, Wang S, Shen X, Li Y, Cheng R, He F. 2022. Antibiotic cocktail-induced gut microbiota depletion in different stages could cause host cognitive impairment and emotional disorders in adulthood in different manners. Neurobiology of Disease. 170:105757. doi:10.1016/j.nbd.2022.105757.
Mosaferi B, Jand Y, Salari AA. 2021. Gut microbiota depletion from early adolescence alters anxiety and depression-related behaviors in male mice with Alzheimer-like disease. Scientific Reports. 11:22941. doi:10.1038/s41598-021-02231-0.