Poly-ADP ribose polymerases (PARPs) are proteins that participate in the process of repairing single strand DNA breaks (SSBs). PARPs carry out surveillance in the cell and when DNA damage occurs, PARPs bind to and recognize DNA damage, setting off DNA repair pathways (Morales et al., 2014). PARP inhibitors (PARPi) block the DNA damage repair pathway, leaving the SSBs unrepaired. Eventually, the SSBs will accumulate and form double stranded breaks (DSBs).
Normal ovarian cells use BRCA proteins to recruit repair complexes to the DSB region and help repair the DSBs using a method called homologous recombination (HR) (Dilmac and Ozpolat, 2023). Homologous recombination is a process in which identical and undamaged nucleotide sequences from homologous chromosomes are used to repair DSBs. In BRCA-mutant ovarian cancer, DSBs are unable to be repaired because BRCA proteins lose function and are unable to recruit repair complexes.
While PARPi is a viable treatment for BRCA-mutant ovarian cancer, 40-50% of BRCA-mutant patients do not respond to PARPi due to innate or acquired PARPi resistance (Dilman and Ozoplat, 2023). Acquired resistance happens because cancer cells reproduce and adapt quickly to overcome the PARPi lethality. In addition, more than 50% of HGSC patients are do not have BRCA mutations, meaning that PARPi treatment are not substantially beneficial to a large majority of patients (Zhang et al., 2023). This highlights the importance of finding a different mechanism by which we can potentiate the therapeutic effect of PARPi treatment. The solution may lie in polyploid giant cancer cells (PGCCs).
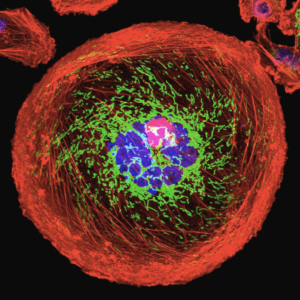
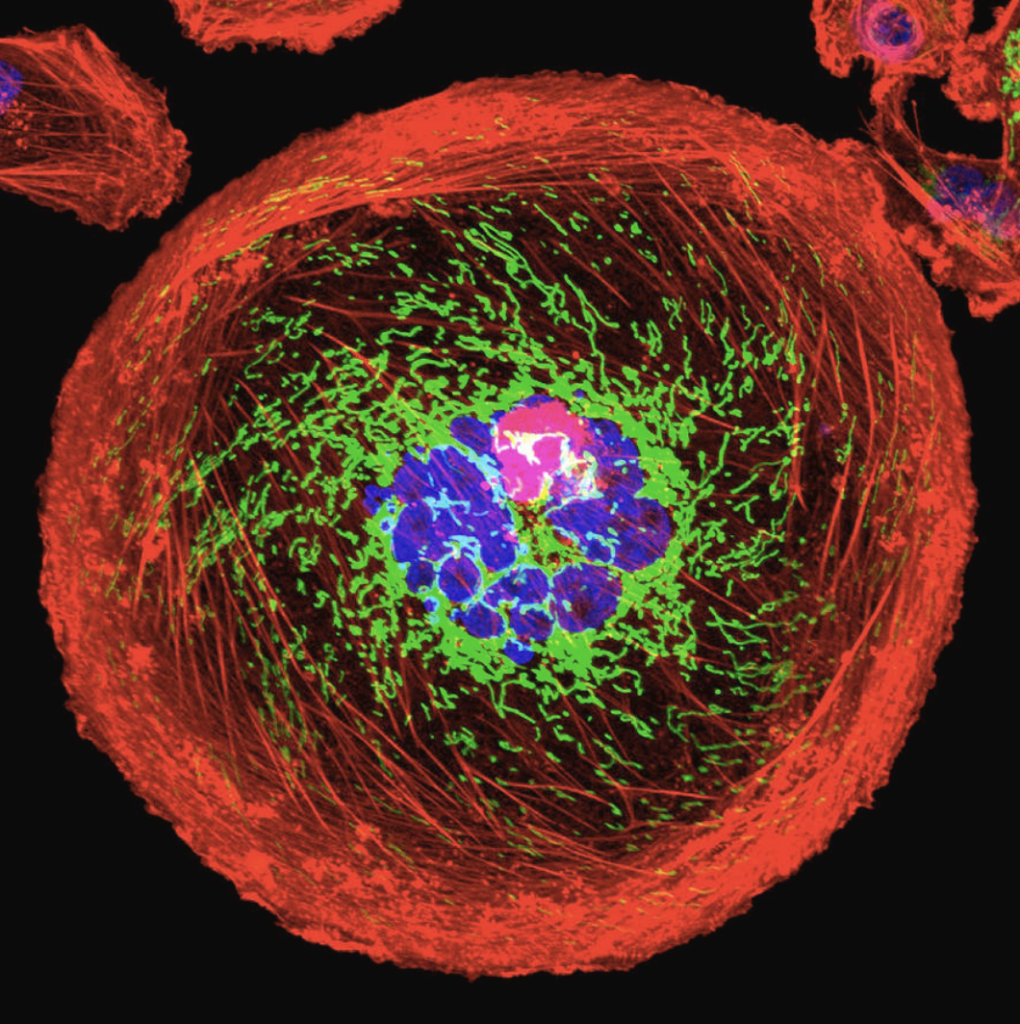
Source: University Of Pittsburgh Cancer Institute. “Polyploid Giant Cancer Cell From Breast.” Photograph. Science Photo Gallery.
PGCCs are large cancer cells that are usually thought to be senescent, meaning that the cells age but do not die or divide. However, scientists are not entirely certain on the formation or function of PGCCs (Zhou et al., 2022).
In Targeting polyploid giant cancer cells potentiates a therapeutic response and overcomes resistance to PARP inhibitors in ovarian cancer, Zhang et al. investigates the association of PGCCs with the PARPi (specifically, Olaparib) resistance in ovarian cancer. Their research looks at high-grade serous carcinoma (HGSC), the most common subtype of ovarian cancer. First, they found that PGCCs grow in Olaparib-resistant HGSC patient-derived xenografts (PDX) and that they were able to induce PGCC formation using Olaparib. PDX is a model of cancer where cancer tissues are collected from patients and planted into an immunodeficient mouse. Second, while PGCCs are usually thought to be senescent, they found that PGCCs are also able to escape senescence and produce daughter cells that are able to divide. A portion of these mitotic competent daughter cells also have therapeutic resistance, increasing PARPi resistance within the tumor. So far, Zhang et al. found that not only can PGCCs become resistant to PARPi treatment, but they can also produce more cancer cells that are also resistant to PARPi treatment. However, this isn’t all bad news.
From previous studies, we know that PGCCs dedifferentiate and mimic early embryonic development (Niu et al., 2017). Leveraging this knowledge, Zhang et al. reasoned that certain contraceptives, such as Mifepristone, may be able to block the life cycle of PGCCs. Indeed, they found that Mifepristone was able to suppress tumor growth in both Olaparib-naive and Olaparib-resistant HGSC cells, treating the original issue of PGCC and daughter cells being resistant to Olaparib. The results showed that PGCCs are most sensitive to a combination of Olaparib and Mifepristone treatment. In conclusion, Zhang et al. found a way to grow PGCCs in HGSC cells and then use the PGCCs to suppress cancer growth while also overcoming PARPi resistance.
Zhang et al. mentioned that PGCCs can be used to potentiate the therapeutic response of PARPi treatment and combat more than one HGSC subtype. Previously, PARPi treatment worked best with BRCA mutant patients. However, Zhang et al. suggest that PGCCs, rather than the BRCA status of tumor, are associated with acquired Olaparib resistance. This means that PGCCs can be used as an alternative treatment regime, broadening applications of PARPi to HGSC patients who do not have BRCA mutations.
References:
Dilmac, S., & Ozpolat, B. (2023). Mechanisms of PARP-Inhibitor-Resistance in BRCA-Mutated Breast Cancer and New Therapeutic Approaches. Cancers, 15(14), 3642. https://di.org/10.3390/cancers15143642
Morales, J. C., Li, L., Fattah, F. J., Dong, Y., Bey, E. A., Patel, M., Gao, J., & Boothman, D. A. (2014). Review of Poly (ADP-ribose) Polymerase (PARP) Mechanisms of Action and Rationale for Targeting in Cancer and Other Diseases. Critical Reviews in Eukaryotic Gene Expression, 24(1), 15–28.
Niu, N., Mercado-Uribe, I., & Liu, J. (2017). Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene, 36(34), 4887–4900. https://doi.org/10.1038/onc.2017.72
Zhang, X., Yao, J., Li, X., Niu, N., Liu, Y., Hajek, R. A., Peng, G., Westin, S., Sood, A. K., & Liu, J. (2023). Targeting polyploid giant cancer cells potentiates a therapeutic response and overcomes resistance to PARP inhibitors in ovarian cancer. Science Advances, 9(29), eadf7195. https://doi.org/10.1126/sciadv.adf7195
Zhou, X., Zhou, M., Zheng, M., Tian, S., Yang, X., Ning, Y., Li, Y., & Zhang, S. (2022). Polyploid giant cancer cells and cancer progression. Frontiers in Cell and Developmental Biology, 10, 1017588. https://doi.org/10.3389/fcell.2022.1017588