Keywords – chemical bonds, covalent bonds, ionic bonds, ions, free radicals, dissociation, synthesis, stimuli, heterolysis
Free radicals are typically atoms that are most commonly found in diatomic molecules (for example, Oxygen) that are not bonded, so they tend to bond to the first available molecule and are therefore very unpredictable. Free radicals are atoms with unpaired electrons attached. They are commonly found in the body or in the environment. Having unpaired electrons means that they are volatile and can randomly bond to other molecules, creating toxic compounds if they become too abundant.
By contrast, ions are atoms with charges that create much more predictable bonds in nature. Like free radicals, ions have a different number of electrons (negatively charged parts) than protons (positively charged parts). However, ions have paired electrons and occur more naturally in the body. Ions are essential for communication between cells.
The synthetic breakdown – or dissociation – of molecules in solutions or bodily environments often releases free radicals instead of ions. This new study explores the possibility of using energy differently to reduce the release of free radicals in synthetic dissociation. The replacement of free radicals with ions will reduce harm to the body, as well as the environment. This opens up new possibilities for the creation of new medicines and more efficient biofuels.
Heterolysis results in the release of ions rather than free radicals. The Nuerberger and Breder lab conducted experiments that center around the understanding that many molecules can not be dissociated into their respective atomic contents via heterolysis, a process that involves breaking molecular bonds by using two different energetic stimuli rather than one (for example, light and heat rather than one or the other). The challenge here is to balance the use of heat and light to efficiently break down molecules without harmful byproducts like free radicals.
This procedure begins with determining the lambda-max value, which is the optimal wavelength of light that will maximize the absorbance in a certain molecule, for the molecules PhSe, PhSe+, and PhSe- (Breder, 2024). These are charged compounds consisting of a Selenium atom attached to a phenyl group, or a six-Carbon ring with five attached Hydrogen atoms. These molecules were selected to mimic the complexity and size of many biological molecules found in the body.
Next, the researchers determined the amount of energy that could be absorbed from a light source with the calculated lambda-max wavelength. This data was obtained through the use of absorbance sensors, which shine a broad spectrum of light with various wavelengths and can detect which wavelengths are blocked the most by dissolved particles. All substances have a lambda-max value, which is why we are able to see in color. Even samples that appear to be colorless have a slight color to them. The lambda-max value of a sample will typically correspond to its complementary color in the red-blue-green system (for example, the lambda-max value for a green substance will correspond to a shade of purple light).
Once the lambda-max values were determined for each substance, the researchers calculated how much energy was contained in the absorbed light. This value was subtracted from the known amount of energy required to break the Se-C (Selenium-Carbon) bonds. The remaining energy was supplemented in the form of heat, and the molecules in turn dissociated with a much higher frequency of ions and atoms with paired electrons than free radicals (Breder, 2024). Researchers were able to quantify the difference in ion dissociation from free radicals by determining experimental lambda-max values after exposure to the light and heat stimuli and comparing those of the ion products and of the free radical products. (see Figure 1 for comparison)
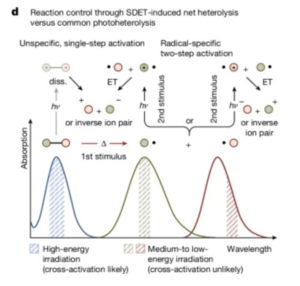
This experiment demonstrates that the combined use of heat and light stimuli to dissociate molecules results in safer byproducts for the human body and the environment. This means that more diverse molecules can be created without extensive energy usage. This leap in chemistry expands into the biological and environmental fields of research, allowing for more complex and efficient medicines, implants, biofuels, plastic replacements, and more to be created.
This research has broad implications in the research world. In environmental sciences, the creation of biofuels can require several steps of synthesizing and dissociating molecules to achieve specific formulas and structures. The knowledge of how to minimize the release of volatile materials during this process is vital to ensuring that living organisms in nearby ecosystems are not harmed by the creation of more renewable fuels.
In medicine, a similar dilemma occurs with the creation of new drugs. While medicinal compounds are synthesized in controlled lab environments, their individual chemical formulas may be counterproductive in that they favor the release of free radicals in the body once administered into such an uncontrolled environment. In addition to reducing the abundance of free radicals in the synthesis of new materials, the knowledge of how to more efficiently construct and break apart molecules opens up new possibilities for entirely unique drug compounds. Ideally, many of these will serve the same functions but will be less structurally in favor of releasing free radicals into the body.
Works Cited
Sayre, Hannah J., and Harsh Bhatia. “Innovative Way to Break Chemical Bonds Broadens Horizons for Making Molecules.” Nature, vol. 632, no. 8025, Nature Portfolio, Aug. 2024, pp. 508–9, https://doi.org/10.1038/d41586-024-02437-y. Accessed 10 Oct. 2024.
Tiefel, Anna F., et al. “Unimolecular Net Heterolysis of Symmetric and Homopolar σ-Bonds.” Nature, vol. 632, no. 8025, Aug. 2024, pp. 550–56, https://doi.org/10.1038/s41586-024-07622-7.