Immortality is biologically possible. It has been biologically possible ever since the discovery of the ‘immortal’ jellyfish Turritopsis. dohrnii in the Mediterranean Sea in 1883. This cnidarian is an exception to the normal cycle of life and death; they have an extra stage in their life cycle known as ‘rejuvenation’ where the mature medusa can metamorphose back into its juvenile form – as polyps. This is usually in response to damage or natural deterioration with age. If they are not eaten or killed by predators, they can rejuvenate and live forever. Therefore there could actually be an existing Turritopsis. dohrnii jellyfish that has lived since the time when dinosaurs roamed the Earth, as this species of jellyfish have been floating in the oceans since 66 million years ago.
Fig 1: The immortal jellyfish: Turritopsis. dohrnii (American Museum of Natural History, 2015)
The diagram on the left of Figure 2 shows what the life cycle of a jellyfish normally is. The fertilized egg first grows into a small larva, known as a planula (Pascual-Torner, 2021). The planula the proceeds to ground itself into a solid surface and form a polyp where it develops a digestive system and reproduces asexually to form a colony (Pascual-Torner, 2021). A section of the polyp within the colony develops a new set of nerves and muscle, which can swim, grow and feed independently; eventually growing into a medusa, a full grown adult jellyfish which can reproduce sexually (Pascual-Torner, 2021). If not by being eaten or injured by a predator, old age is usually what kills these jellyfish (Pascual-Torner, 2021).
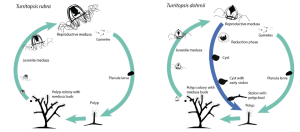
Fig 2: The life cycles of Turritopsis. rubra (‘normal’ lifecycle) and Turritopsis. dohrnii (Pascual-Torner, 2021)
Aging is generally governed by cellular senescence – formally defined as a state of cell cycle arrest where proliferating cells stop responding towards growth-promoting stimuli (Osterloff). This is usually in response to stresses such as telomere dysfunction and persistent DNA damage (Cell Signaling Technology). The number of senescent cells normally increases with age, negatively impacting other biological processes and impairs any potential for pluripotency – ability of a cell to differentiate into any type of specialized cell – and the possibility of regeneration. But this cnidarian species is able to challenge this trait and reverse their life cycle even after reaching sexual maturity, through a process called ontogeny reversal.
Comparative genomic studies have been conducted in hopes to find the genes involved in ontogeny reversal. In the study conducted by Pascual-Torner et al. (2021), the genes involved in aging and DNA repair were compared between Turritopsis. dohrnii and Turritopsis. rubra, which can’t rejuvenate at mature stages. Some of their findings suggested that Turritopsis. dohrnii may have “more efficient replicative mechanisms and repair systems” (Pascual-Torner et al., 2021). This includes the amplification of the genes POLD1 and POLA2, which encode for the enzyme DNA polymerase. This enzyme is involved in DNA replication, therefore its respective genes being amplified suggest enhanced replication in this cnidarian species. Furthermore, duplications to certain DNA repair genes such as XRCC5, GEN1, RAD51C, and MSH2 suggest more efficient DNA repair mechanisms (Pascual-Torner et al., 2021). A more efficient DNA repair mechanism reduces DNA damage and the triggers for cellular senescence, resulting in the slowing down of aging. In addition, there are also many other genomic differences noted by the study that also contribute to reducing the stressors for senescence that are not mentioned in this article.
However, the ability Turritopsis. dohrnii has for ontogeny reversal implies that this jellyfish species additionally possesses some sort of cell reprogramming mechanism. To promote dedifferentiation, where cells grow in reverse from a differentiated stage to a less differentiated stage, there should be pathways that target the enzyme PRC2 (polycomb repression complex 2) and pluripotency related genes (Pascual-Torner et al., 2021). PRC2 catalyzes methylation of a specific set of histones to silence specific genes that enhance and maintain pluripotency in embryonic stem cells (Pascual-Torner et al., 2021). According to the same study, the silencing of PCR2 targets and activation of pluripotency targets was observed in Turritopsis. dohrnii (Pascual-Torner et al., 2021). Through these mechanisms, pluripotency is enhanced, leading the jellyfish to be able to form undifferentiated cells and thereby reversing back into its cyst stage, which is similar to its planula stage, as shown in Figure 2.
It is mind-blowing that a mere floating sea creature is able to reverse its lifecycle and biologically be immortal. There is still a lot unknown about its process to being able to be immortal; scientists have only just started to uncover the basics from their genomic analysis. Maybe as scientists go deeper into uncovering Turritopsis. dohrnii’s strange immortality, we can start to think about whether we can transfer this ability to other creatures, even humans – or if we even want to? Now that immortality is actually a reality, do we want it to be a biological possibility for other creatures, for us?
References
“Cellular Senescence.” Cell Signaling Technology, https://www.cellsignal.com/science-resources/overview-of-cellular-senescence.
Matsumoto, Yui, and Maria Pia Miglietta. “Cellular Reprogramming and Immortality: Expression Profiling Reveals Putative Genes Involved in Turritopsis Dohrnii’s Life Cycle Reversal.” Genome Biology and Evolution, edited by Dennis Lavrov, vol. 13, no. 7, July 2021, p. evab136. DOI.org (Crossref), https://doi.org/10.1093/gbe/evab136.
Osterloff, Emily. “Immortal Jellyfish: The Secret to Cheating Death.” Natural History Museum, https://www.nhm.ac.uk/discover/immortal-jellyfish-secret-to-cheating-death.html.
Pascual-Torner, Maria, et al. “Comparative Genomics of Mortal and Immortal Cnidarians Unveils Novel Keys behind Rejuvenation.” Proceedings of the National Academy of Sciences, vol. 119, no. 36, Sept. 2022, p. e2118763119. DOI.org (Crossref), https://doi.org/10.1073/pnas.2118763119.
“The ‘Immortal’ Jellyfish That Resets When Damaged: AMNH.” American Museum of Natural History, 2015, https://www.amnh.org/explore/news-blogs/on-exhibit-posts/the-immortal-jellyfish.