By 2023, 81.4% of the United States population have received at least one dose of the COVID-19 mRNA vaccine, and just about everyone has heard of these lifesaving shots (CDC, 2023). But how many of us actually know how they work? In general, vaccinations expose the body to a weakened strain of a virus that initiates an immune response so that the virus can be recognized, and quickly neutralized, in the future (Jain, 2021). This works because of the structure of our immune system, which is split up into innate immunity and adaptive immunity. Innate immunity is the immediate response that our bodies put up when exposed to a pathogen; it sends out macrophages to gobble up infected cells, creates an inflammatory response to kill off the infection, and uses a host of molecules and pathways to destroy the virus. Adaptive immunity involves the presence of B and T cells, which arise in a viral infection and create immunological memory through antibodies and memory cells so that a future infection of the same virus is immediately recognized and shut down. Vaccines work to stimulate this adaptive immunity, while minimizing innate immunity.
mRNA vaccines use this same basic principle, but instead of injecting a weakened strain of the actual virus into someone, they instead inject a piece of mRNA, or messenger RNA, which codes for a viral protein that stimulates this immune response (Jain, 2021).
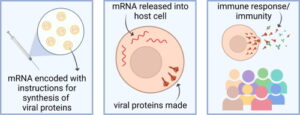
Figure 1: The methodology of mRNA vaccines. Adapted from Jain, et. al., 2021
mRNA vaccines are much easier to make than traditional vaccines because they don’t require growing and inactivating a virus, and they don’t carry the risk that live vaccines have of infecting the subject, but up until recently, they weren’t even remotely possible because in vitro mRNA would create a huge inflammatory response when entering the body (Karikó, 2005). However, Hungarian biochemist Katalin Karikó and her colleague Drew Weissman were undeterred, and their research to create mRNA without an inflammatory response paved the way for mRNA vaccines and won them the Nobel Prize in Medicine in 2023 (Karikó, 2005).
Karikó and Weissman’s research focused on in vitro mRNA’s stimulation of Toll-like receptors (TLRs), which recognize pathogens and activate signaling pathways leading to an inflammatory response (Karikó, 2005). They discovered that mammalian mRNA, which doesn’t stimulate an immune response, contains many base pair modifications, whereas bacterial RNA, which is known to stimulate a response, has no modifications (Karikó, 2005). In vitro mRNA, grown in a lab rather than from mammalian cell cultures, also has no, or very few, modifications. This led the researchers to question whether base pair modifications were how cells distinguished between foreign and non-foreign RNA, and thus which pieces of RNA stimulate an immune response (Karikó, 2005). They discovered that in vitro RNA activates three main TLRs known for activating an inflammatory response, but that nucleoside modifications limit that activation. Most importantly, they discovered that the suppressed immune response is proportional to the number of nucleotide modifications, and that these modifications also increase the stability of in vitro mRNA in the body, another original roadblock in the usage of mRNA for vaccines. (Karikó, 2005).

Figure 2: The use of base modifications to suppress inflammatory response of RNA. Adapted from Nobelprize.org
Combined with later research also by Karikó and Weissman discovering that modified base-pairs also create increased protein production and other research designing more stable lipid carriers to deposit the mRNA into human cells, mRNA became a promising new vaccine technology, eventually allowing the COVID-19 vaccines to be produced in record times, shortening the pandemic and granting all of us our lives back. But the road to Karikó’s research being recognized, and especially the road to the Nobel Prize, was not predetermined. Karikó’s research was long overlooked, in part because of her identity as a Hungarian immigrant. She was “demoted four times” at UPenn, being told that her research didn’t measure up to their standards, but she continued to persevere, making her both a scientific success story, and a personal one (Shrikant, 2023). The COVID-19 vaccines created by Pfizer and Moderna were the first mRNA vaccines to be released, but future research is focusing on creating a universal flu vaccine and possibly even an HIV vaccine, all using mRNA technologies, which could drastically improve the landscape of viral infections, both in the US and across the globe.
Literature Cited
Karikó, K., Buckstein, M., Ni, H., & Weissman, D. (2005). Suppression of RNA Recognition by Toll-Like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity, 23(2), 165–175. https://doi.org/10.1016/j.immuni.2005.06.008
Karikó, K., Muramatsu, H., Welsh, F. A., Ludwig, J., Kato, H., Akira, S., & Weissman, D. (2008). Incorporation of Pseudouridine into mRNA yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Molecular Therapy, 16(11), 1833–1840. https://doi.org/10.1038/mt.2008.200
Jain, S., Venkataraman, A., Wechsler, M. E., & Peppas, N. A. (2021). Messenger RNA-Based Vaccines: Past, Present, and Future Directions in the Context of the COVID-19 Pandemic. Advanced Drug Delivery Reviews, 179, 114000. https://doi.org/10.1016/j.addr.2021.114000
The Nobel Prize in Physiology or Medicine 2023. (n.d.). NobelPrize.Org. Retrieved October 22, 2023, from https://www.nobelprize.org/prizes/medicine/2023/press-release/
CDC. (2020, March 28). Covid Data Tracker. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker
Shrikant, A. (2023, October 6). Nobel Prize Winner Katalin Karikó was “demoted 4 times” at her old job. How she persisted: “You have to focus on what’s next.” CNBC. https://www.cnbc.com/2023/10/06/nobel-prize-winner-katalin-karik-on-being-demoted-perseverance-.html