Inborn protective mechanisms present challenges for therapies targeting cancers of the brain. Engineered nanoparticles permit the selective delivery of CRISPR-Cas9 to glioblastoma tumors.
Glioblastoma accounts for almost half of all cancerous tumors originating in the brain.1 Even with maximum safe treatment, the median survival period for patients is less than 1.5 years.2 However, survival varies widely by age, from a 0.9% 5-year survival rate for patients over 75 years old to an 18.2% 5-year survival rate for patient 0-19 years old.3 Nevertheless, the overall 5-year survival rate remains low at 5%2 and novel therapies are urgently needed for glioblastoma treatment. Zou et al. present a highly specific CRISPR-Cas9-based gene therapy for glioblastoma.4
Gene therapies offer an attractive treatment for cancers. The goal of gene therapy is to mutate or remove deleterious DNA sequences such that they are unable to be transcribed and translated into functioning proteins. In the most prevalent technique, CRISPR-Cas9, single guide RNA (sgRNA) identifies and binds to the target DNA sequence. It then recruits Cas9 proteins to excise parts of the target DNA sequence. Mutations are generated as the cell tries to repair the damaged DNA.5
While a powerful tool, researchers have struggled to effectively deliver CRISPR-Cas9 to their cellular target. Delivery is particularly complex in brain tumors such as glioblastoma. Traditionally medications are trafficked through the body and delivered to their target through the bloodstream. However, the brain is a more complex and protected system. Primarily, selective delivery of therapeutics to tumor cells is necessary to protect neuronal function. Moreover, the brain is separated from the blood stream by a thin layer of cells termed the blood-brain barrier (BBB). The BBB allows for selective permeation of compounds into the brain, shielding neurons from toxins while permitting the passage of essential nutrients.6 Previous studies have sought to transport CRISPR-Cas9 therapies across the BBB using viruses as delivery capsules7 or circumvent the BBB altogether via intercranial injection of therapeutics.8 Yet these methods carry risk, either an immune response to the viral vector or complications from the invasive injection.
Zou et al. sought to resolve the issues of specific cell targeting and BBB permeability in CRISPR-Cas9 delivery by encapsulating the gene editing complex within a nanoparticle. The researchers chose to target Polo-like kinase 1 (PLK1) using CRISPR-Cas9 gene therapy. PLK1 is an attractive for selective gene therapy due to its higher overexpression by glioblastoma cells and by the proliferative glioblastoma subtype in particular.9 Moreover, inhibition of PLK1 has been shown to reduce tumor growth and induce cell death.9
The researchers encapsulated Cas9 and sgPLK1 in a neutrally charged nanoparticle for delivery. The small size of nanoparticles, which are measured in nanometers, allow for easy transport through the bloodstream and uptake by cells. Taking advantage of the high expression of lipoprotein receptor-related protein-1 (LRP-1) on both BBB endothelial cells and glioblastoma tumor cells, they decorated the nanocapsule surface with LRP-1 ligand angiopep-2 peptide to facilitate selective uptake by BBB and glioblastoma cells (Figure 1). Zou and her colleagues bound the nanoparticle together with disulfide bonds as an added layer of selectivity for glioblastoma cell delivery. In the high glutathione environment of a glioblastoma cell, the nanoparticle dissolves, releasing its contents. However, glutathione concentrations are lower in BBB endothelial cells and healthy neurons, reducing the dissolution of the nanoparticles and leaving healthy DNA alone.
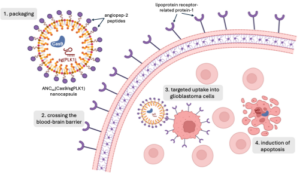
Figure 1. Nanoparticles enable permeation of the blood brain barrier (BBB) and selective delivery of the CRISPR/Cas9 system to glioblastoma (GBM) cells. Angiopep-2 peptides, which decorate the nanoparticle’s surface, bind with lipoprotein receptor-related protein-1 (LRP-1) overexpressed on BBB and GBM cells. Following uptake into GBM cells, the nanoparticles dissolve in the high glutathione environment, releasing the Cas9 nuclease and single guide RNA (sgRNA) targeting Polo-like kinase 1 (PLK-1) genes. Zou et al. demonstrated the selective mutation of PLK-1 induced apoptosis in GBM cells with minimal off-target effects.
Through Cas9/sgPLK1 delivery by nanoparticle, Zou et al. demonstrated a 53% reduction in expression of the targeted gene in vitro. PLK1 gene editing was cell selective, with negligible genetic mutation in the healthy surrounding brain tissue. While nanoparticles with and without disulfide cross-linking were capable of gene editing, the disulfide cross-linked nanoparticle induced almost 400% more mutations. Glioblastoma cells treated with disulfide cross-linked nanoparticles were also over 3 times more likely to undergo apoptosis cell death. Mice grafted with patient glioblastoma tumors treated with Cas9/sgPLK1 nanocapsules experienced an almost 3-fold extension in life expectancy, suggesting this treatment as a viable anti-glioblastoma therapy.5
Yet in order to be effectively applied as a cancer therapy, the efficiency of this nanoparticle delivery system must be increased. In mouse glioblastoma models, Zou et al. only achieved a maximum accumulation of 12% and effected a 38% knockdown of PLK1.5 Despite their low magnitude, these values are far greater than similar gene therapy treatments currently studied, suggesting nanoparticles present an innovation in the delivery of CRISPR-Cas9 to difficult to access tumors.
In addition to the improved efficacy over existing systems, the work of Zou et al. opens the door for less invasive administration of treatment. In contrast to previous gene therapies administered directly to the tumor through intercranial injection, the BBB penetration and tumor-specific accumulation of the nanoparticles may permit systemic administration. Zou et al. injected the nanoparticles intravenously, but treatment may even be given as a pill taken orally, eliminating any surgical intervention. Moreover, due to their modular design, the engineered nanoparticles may be adapted as targeted treatments for other tumors. Exchanging the angiopep-2 peptides for another ligand would facilitate uptake by cells expressing the corresponding receptor. The load carried within the nanoparticle could be altered to contain a different sgRNA targeting a new gene or another therapeutic entirely as a complementary treatment. Nanoparticle delivery systems like that studied by Zou et al. contains many layers of selectivity, offering hope for effective delivery of treatment to previously inaccessible tumors.
Works Cited:
- Wirsching, H.-G. & Weller, M. Glioblastoma. in Malignant Brain Tumors : State-of-the-Art Treatment (eds. Moliterno Gunel, J., Piepmeier, J. M. & Baehring, J. M.) 265–288 (Springer International Publishing, Cham, 2017). doi:10.1007/978-3-319-49864-5_18.
- Delgado-López, P. D. & Corrales-García, E. M. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 18, 1062–1071 (2016).
- Ostrom, Q. T. et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol 16, iv1–iv63 (2014).
- Lino, C. A., Harper, J. C., Carney, J. P. & Timlin, J. A. Delivering CRISPR: a review of the challenges and approaches. Drug Delivery 25, 1234–1257 (2018).
- Zou, Y. et al. Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci Adv 8, eabm8011.
- Dotiwala, A. K., McCausland, C. & Samra, N. S. Anatomy, Head and Neck: Blood Brain Barrier. in StatPearls (StatPearls Publishing, Treasure Island (FL), 2024).
- Song, R. et al. Selection of rAAV vectors that cross the human blood-brain barrier and target the central nervous system using a transwell model. Molecular Therapy Methods & Clinical Development 27, 73–88 (2022).
- Lee, B. et al. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat Biomed Eng 2, 497–507 (2018).
- Lee, C. et al. Polo-Like Kinase 1 Inhibition Kills Glioblastoma Multiforme Brain Tumor Cells in Part Through Loss of SOX2 and Delays Tumor Progression in Mice. Stem Cells 30, 1064–1075 (2012).


