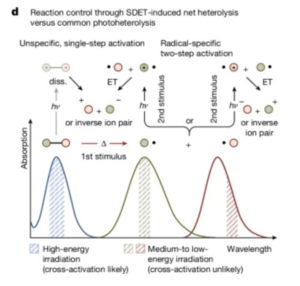
People often think the most important aspect of how exercise affects your overall health is how hard you work, how much weight you can lift, or how far you can run. However, two recent studies have uncovered another factor that might be just as important for maximizing health benefits – when you exercise. Multiple studies have looked at the impact of how when you exercise affects your body. For example, one study looked at the impact of exercise timing in mice, focusing on the growth of muscle tissue, while another study looked at a large population of people and how their exercise habits affected sleep quality. Together, these studies show that when exercise takes place matters more than most people believe.
The first study, done by Liu et al. and published in Nature Communications, looked at how timing of exercise in mice affected long-term health (Liu et al., 2025). Mice, like people, have a circadian rhythm, which is a 24-hour internal clock in the body that regulates and affects energy, metabolism, and sleep. Muscles in the body also have internal clocks, which decide when to burn fat or sugar.
In the study, Liu et al. had two groups of mice run at a low intensity and low volume on treadmills at different times of day: one group exercised before sleep and the other exercised right after waking up. Training lasted for several months, and researchers measured the mice’s body weight throughout the study and measured the mice’s strength, endurance, and blood sugar before and after the study was conducted, all of which are indicators of long-term exercise results. The results of the study were quite clear. Mice who exercised before sleep showed increased physical and metabolic improvements after the period of consistent exercise, meaning they gained less fat, had more endurance, and showed better blood sugar control. The group of mice that exercised after waking saw less improvement in these areas (Liu et al., 2025).
The second study, done by Leota et al. and also published in Nature Communications, tracked the health data of over 14,000 human participants using fitness wearables over four million nights of sleep (Leota et al., 2025). The researchers wanted to see whether exercising in the evening, before bedtime, affected sleep quality.
The researchers found that the later and harder people worked out, the more their sleep was affected. When people exercised four or more hours before going to bed, their sleep was normal, regardless of the intensity of the workout. When people exercised two to four hours before going to bed, they took a longer time to fall asleep and slept less. When people exercised two hours or less before going to bed, especially at a high intensity, sleep noticeably got worse. Some took up to over an hour longer to fall asleep, slept about 40 minutes less overall, and had a higher heart rate throughout the night (Leota et al., 2025).
Although the mice study found that exercise before bed improved overall health, the human study found that the closer exercise got to bedtime, the worse sleep became, which is also known to negatively impact recovery and overall health. While these studies may seem contradictory, they actually align upon consideration of the factor of exercise intensity. High-intensity training in the evening negatively affects sleep, while low/moderate-intensity exercise in the evening is beneficial for muscle growth and recovery without impacting sleep.
Although both studies were different, they arrived at the same key conclusion: that the body works best when its internal cycles, like its circadian rhythm, are not disrupted. Exercise, such as heavy lifting or sprinting, activates the body’s sympathetic nervous system, which is the part of the nervous system responsible for the “fight or flight” response. Sleep, along with recovery, lowered heart rate, and relaxation, is activated by the parasympathetic nervous system, otherwise known as the “rest and digest” state. While the activation of the sympathetic nervous system is good for exercise and performance, it is not good when the body needs to sleep. Instead of letting the body settle down, activation of the sympathetic nervous system keeps your body revved up, lessening sleep time and quality, and therefore overall recovery and future performance.
Because of the busyness of daily life, it’s not always possible to perfectly time every workout. Evening workouts are often unavoidable due to the realities of many people’s daily schedules. However, the combination of results from these studies shows that evening workouts aren’t automatically bad for overall health. In fact, they can even improve the benefits of exercise as long as their intensities are adjusted according to their relation to bedtime. If working out in the evening more than four hours before bedtime, high-intensity exercise can take place without risk of impacting sleep quality and physical health. If working out four hours or less before bedtime, it is better to opt for lower-intensity exercise, which will allow you to sleep better and recover more quickly. In the end, both studies show that being slightly more intentional about when and how hard you train can make a real difference in your sleep, recovery, and overall performance.
Works Cited
Liu, J., Xiao, F., Choubey, A., Kumar S, U., Wang, Y., Hong, S., Yang, T., Otlu, H. G., Oturmaz, E. S., Loro, E., Sun, Y., Saha, P., Khurana, T. S., Chen, L., Hou, X., & Sun, Z. (2025). Muscle Rev-erb controls time-dependent adaptations to chronic exercise in mice. Nature Communications, 16(1), 5708. https://doi.org/10.1038/s41467-025-60520-y
Leota, J., Presby, D. M., Le, F., Czeisler, M. É., Mascaro, L., Capodilupo, E. R., Wiley, J. F., Drummond, S. P. A., Rajaratnam, S. M. W., & Facer-Childs, E. R. (2025). Dose-response relationship between evening exercise and sleep. Nature Communications, 16(1), 3297. https://doi.org/10.1038/s41467-025-58271-x