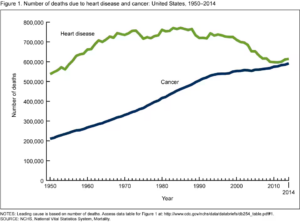
Microplastic pollution is a global issue effectively impacting all aquatic systems from the poles to tropical reefs. Current emission patterns project to around 35 – 98 metric tons of annual microplastic emission by 2030. Yet, this may only be an underestimation, as our current understanding of microplastic concentrations based on traditional sampling practices overlooks smaller debris (Lindeque et al. 2020, Borrelle et al. 2020). With this scale of rapid increase in concentrations, the implications of microplastic accumulation in marine systems have become an increasing concern. In response to this global concern, Adam Porter and his team looked towards the ocean’s floor to better understand how microplastics interact with dynamic ecosystems.
Microplastics emitted into the marine environment can adversely impact a wide range of processes from cellular metabolism to digestive functions, fertility, locomotion, and growth (Foley et al. 2018; Bour et al. 2018). Furthermore, bioaccumulation, or trophic transfer when contaminated prey is consumed by predators, magnifies microplastic burdens in organisms higher in the food chain. These above properties, in conjunction to the rapidly increasing environmental concentrations, highlight the pressing need to quantify how much microplastics marine organisms are ingesting.
Historically, our understanding of individual microplastic burdens has often assumed that levels of environmental contamination directly map onto their uptake by marine organisms. However, studies have found that this isn’t always the case. Other factors, such as feeding strategies and community composition, also impact a species’ uptake rate (Pagter et al. 2021; Bour at al. 2018).
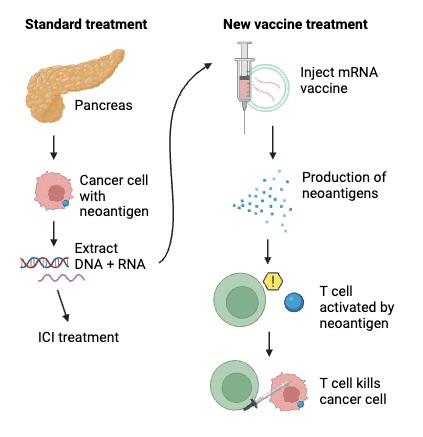
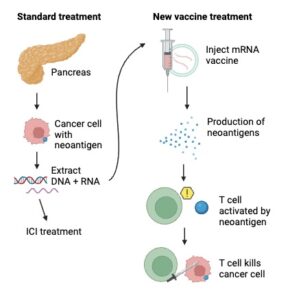
To bridge the mismatch of environmental concentration and individual burden, Porter et al. reviewed 412 studies on marine invertebrates from around the globe to investigate how different species traits could influence microplastic uptake. First, they gathered data from each study and assigned a geographic sector to each sampling site. Next, they evaluated each observation for a variety of variables, including feeding mode, position within the sediment, and wet weight (mass) of the individual. Then, Porter’s team used statistical tests to examine the potential influence each parameter had on plastic uptake with statistical analyses tests and visualized their findings.
Geographically, the Pacific Northwest, Yellow Sea and Japan Trench, had the highest mean individual microplastic burden. In terms of animal class, the highest mean burden occurred in the Malacostraca class. Malacostraca encompasses common commercial species such as crabs and lobsters, which could have commercial implications on industries like lobster fishing and aquaculture.
Of all the outlined parameters, feeding strategies had the greatest impact on microplastic uptake. Omnivores were shown to have the highest rate of uptake, followed by predators, herbivores, grazers, suspension feeders, deposit feeders, and lastly scavengers. These findings support the bioaccumulation theory, one of several hypotheses concerning microplastic uptake patterns (Wang 2014). According to the bioaccumulation theory, microplastics enter the food web through primary consumers like suspension feeders, grazers, and filter feeders. The plastic they retain in their systems will then be ingested by higher trophic levels like secondary and tertiary consumers that are omnivores,predators, and scavengers. Accordingly, the microplastic burdens would be highest in predators and omnivores, which matches the study’s findings.
In addition to the quantity of microplastics retained, feeding patterns were also found to influence the size and type of microplastics consumed were also different across groups. The most reported shape was fibers. The mean sizes of these fragments ranged from 0.2 micrometers to 17 centimeters, and herbivores in general retained the largest particles, but the precise mechanisms driving these patterns remain unclear.
These findings precisely highlight our gap in knowledge of microplastic distribution amongst marine communities. As Porter et al. highlights, a holistic consideration of subtle processes related to feeding patterns is essential in fine tuning our understanding of how our world is changing. Thus, although the study describes general trends on a global scale, future research focusing on regional subtleties is important. Subsequently, applying these findings as policy is crucial, as many marine organisms are frequently consumed commercial species. Being major consumers of seafood, the microplastic accumulation in marine animals can directly impact humans. This is particularly concerning in context of our status as the apex predator, and therefore the final stop in the chain of bioaccumulation. As the microplastic burden in marine organisms is rising at an alarming pace, the need for action is more urgent than ever.
Works Cited
Borrelle, S. B., Ringma, J., Law, K. L., Monnahan, C. C., Lebreton, L., McGivern, A., Murphy, E., Jambeck, J., Leonard, G. H., Hilleary, M. A., Eriksen, M., Possingham, H. P., De Frond, H., Gerber, L. R., Polidoro, B., Tahir, A., Bernard, M., Mallos, N., Barnes, M., & Rochman, C. M. (2020). Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science, 369(6510), 1515–1518. https://doi.org/10.1126/science.aba3656
Bour, Agathe, Carlo Giacomo Avio, Stefania Gorbi, Francesco Regoli, and Ketil Hylland. “Presence of Microplastics in Benthic and Epibenthic Organisms: Influence of Habitat, Feeding Mode and Trophic Level.” Environmental Pollution (Barking, Essex: 1987) 243, no. Pt B (December 2018): 1217–25. https://doi.org/10.1016/j.envpol.2018.09.115.
Foley, Carolyn J., Zachary S. Feiner, Timothy D. Malinich, and Tomas O. Höök. “A Meta-Analysis of the Effects of Exposure to Microplastics on Fish and Aquatic Invertebrates.” The Science of the Total Environment 631–632 (August 1, 2018): 550–59. https://doi.org/10.1016/j.scitotenv.2018.03.046.
Lindeque, P. K., Cole, M., Coppock, R. L., Lewis, C. N., Miller, R. Z., Watts, A. J. R., Wilson- McNeal, A., Wright, S. L., & Galloway, T. S. (2020). Arewe underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environmental Pollution, 265, 114721. https://doi.org/10.1016/j.envpol.2020.114721
Pagter, Elena, Róisín Nash, João Frias, and Fiona Kavanagh. “Assessing Microplastic Distribution within Infaunal Benthic Communities in a Coastal Embayment.” Science of The Total Environment 791 (October 15, 2021): 148278. https://doi.org/10.1016/j.scitotenv.2021.148278.
Porter, A., Godbold, J. A., Lewis, C. N., Savage, G., Solan, M., & Galloway, T. S. (2023). Microplastic burden in marine benthic invertebrates depends on species traits and feeding ecology within biogeographical provinces. Nature Communications, 14(1), 8023. https://doi.org/10.1038/s41467-023-43788-w
Wang, W. -X. “Chapter 4 – Bioaccumulation and Biomonitoring.” In Marine Ecotoxicology, edited by Julián Blasco, Peter M. Chapman, Olivia Campana, and Miriam Hampel, 99–119. Academic Press, 2016. https://doi.org/10.1016/B978-0-12-803371-5.00004-7.



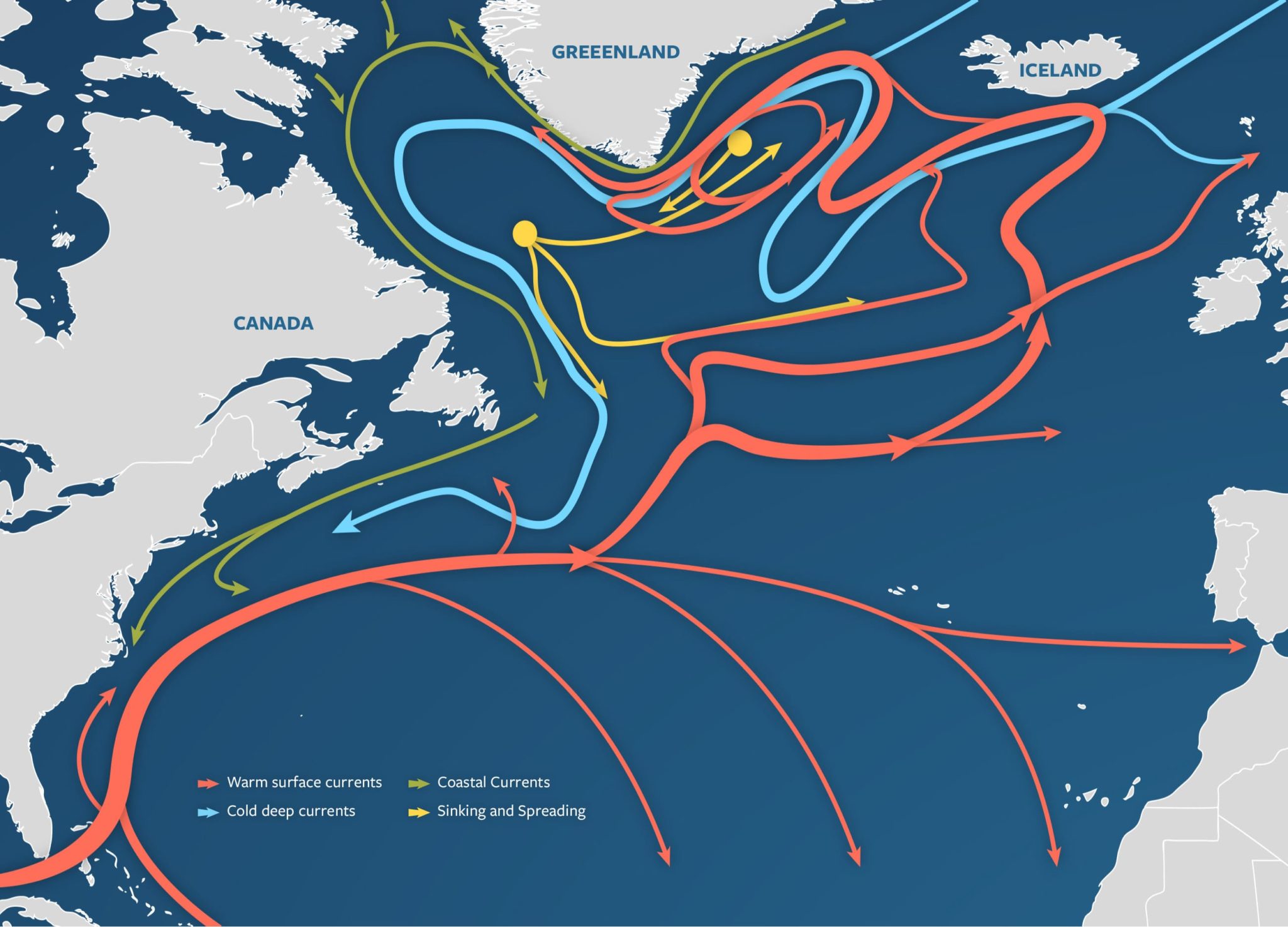 Figure 1: A visualization of the Atlantic Meridional Overturning Circulation (Adapted from “The Ocean Conveyor – Woods Hole Oceanographic Institution,” n.d.).
Figure 1: A visualization of the Atlantic Meridional Overturning Circulation (Adapted from “The Ocean Conveyor – Woods Hole Oceanographic Institution,” n.d.). Figure 2: AMOC strength at 1000m depth and 26° N latitude. Yellow band shows the range of previously observed AMOC strength (Adapted from Van Westen et al., 2024).
Figure 2: AMOC strength at 1000m depth and 26° N latitude. Yellow band shows the range of previously observed AMOC strength (Adapted from Van Westen et al., 2024).