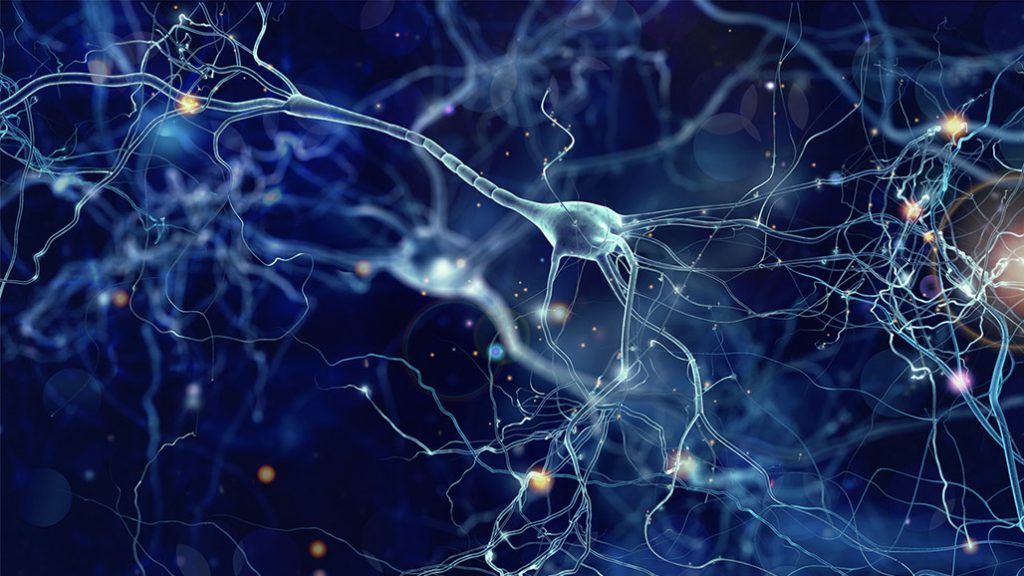
The human brain is organized into cortices, lobes, hemispheres, and more, with every designation serving as a location where a particular function necessary for survival is hosted. In understanding the cell types of the brain, scientists can further shape the understanding of the nature of human life. Current work strives toward comprehending the functions and capacities of the brain and developing stronger foundations for modeling brain physiology to support future research and medical applications. All of the advancements discussed originated from Alyssa Weninger and Paola Arlotta’s Science review article, A family portrait of human brain cells, which compiles recent findings in brain mapping research as aligned with the National Institute of Health’s BRAIN Initiative. In the article, Weninger and Arlotta summarize and discuss the work of multiple groups of neuroscientists that have developed new findings about the brain’s composition and variability across regions, individuals, and species (specifically five primates of interest and mice).
As suggested by the article, recent research from multiple teams of neuroscientists utilized a variety of study mechanisms to compare the composition of the brain. One of the most important tools used in the studies included single-cell profiling. This profiling technique analyzes cellular behavior through multiple methods that include their transcriptome (range of genetic information produced to control cell behavior), proteome (range of proteins produced by the cell), and epigenome (range of modifications and markings that control the genetic information expressed by a cell) to organize them into groups based on their functional similarities. Models that encompass these methods and human organoids (structures of organs derived from STEM cells that mimic organ tissue) are developed to model the brain and its cells. They are also used in mapping and developing comparative analyses to determine significant findings and understanding of the brain organization.
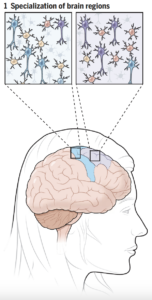 Comparisons of cell composition in regions across the brain resulted in findings from researchers under Siletti from the University of North Carolina at Chapel Hill and Jorstad from Harvard University. The two groups found that rather than mainly having different types of cells in different parts of the brain, some different parts of the brain shared the same cells but had different proportions of these cells. There were some exceptions, such as inhibitory neurons in the primary visual cortex, although the explanation of this finding is unclear. Such results change the understanding of evolutionary diversity in that diversification does not depend heavily on having many different cell types, but rather on having varying proportions of cells with small differences.
Comparisons of cell composition in regions across the brain resulted in findings from researchers under Siletti from the University of North Carolina at Chapel Hill and Jorstad from Harvard University. The two groups found that rather than mainly having different types of cells in different parts of the brain, some different parts of the brain shared the same cells but had different proportions of these cells. There were some exceptions, such as inhibitory neurons in the primary visual cortex, although the explanation of this finding is unclear. Such results change the understanding of evolutionary diversity in that diversification does not depend heavily on having many different cell types, but rather on having varying proportions of cells with small differences.
Jorstad’s group also developed a significant result in identifying differences in brain composition between human individuals. One cell type from 75 individuals was profiled and resulted in different classes of cells bearing contrasting levels of variability among individuals. Most of the explanatory factors were beyond demographic differences, such as gender, ancestry, or age. The reason for such differences is still unclear. Scientists are further encouraged to study bigger cohorts of people to further examine the origin of differences in variability across humans.
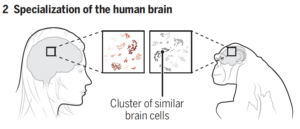
The finding of varying cell proportions held as Jorstad’s group conducted interspecies comparisons, comparing human compositions with other primates (specifically chimpanzees, gorillas, rhesus macaques, and marmosets). The exceptional cognitive ability found in humans was largely supported by differences in proportions of brain cell types rather than the variability of cell types. Additionally, faster evolutionary divergence may explain the differences in gene expression found between supportive tissue, known as glial cells, in the brain. This allowed for further species-specific development across primates. Only a limited number of gene patterns specific to humans were found, most of them concentrated in parts of the brain with human evolutionary change. As such, scientists have come to understand that attributes of the human brain are derived from very few cellular or molecular changes, leaving differences in cell proportions as the most prominent explanatory factory for human brain development. Furthermore, understanding the brains of related primates and their relation to human brains will help scientists develop new models for brain pathways and understand the kinds of questions that they will be able to answer with such knowledge in the future.
Neuroscientists today continue to work hard toward developing human brain models. Current studies are focusing on developing accurate organoids – three-dimensional tissue models of stem cells developed to mimic organs in structure and function. Velmeshev’s group of researchers worked towards profiling different cortical (outer layers of the upper brain) areas and related areas in fetuses to track developments across human births. Kim’s group of researchers investigated single-cell transcriptomes of the thalamus (the processor of sensory data) during its development but was missing an investigation of the thalamus cellular compositions. The work of these scientists contributes to the idea of molecular mechanisms as the driver of cellular diversity in the brain, but also calls for more innovation in external biological investigations to better model the brain and further study its composition. In doing so, neuroscientists will come even closer to understanding one of the most complex systems in the human body and develop more answers for current-day neurological problems.
Bibliography
Weninger, Alyssa, and Paola Arlotta. “A Family Portrait of Human Brain Cells.” Science, vol. 382, no. 6667, Oct. 2023, pp. 168–69, https://doi.org/10.1126/science.adk4857.
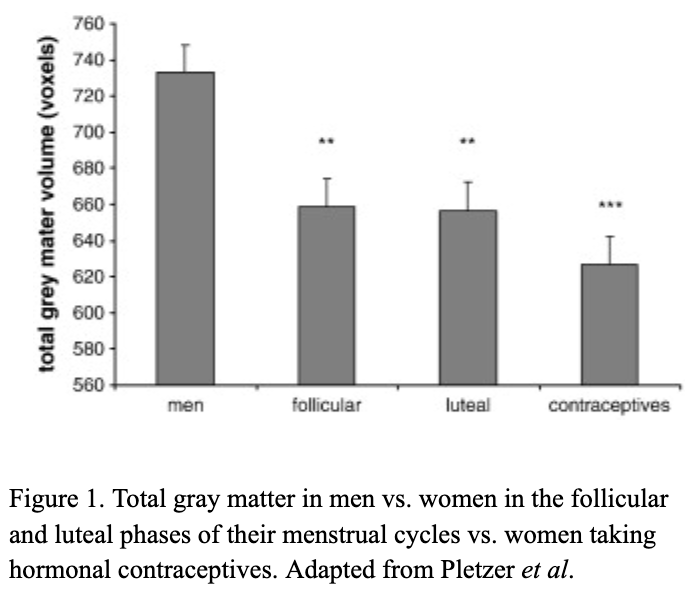
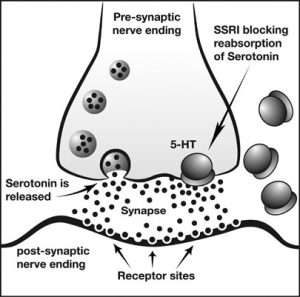
 There are millions of women taking steroids every day. But how is this possible? Are they just getting really buff? It feels like we always hear stories about how performance-enhancing drugs, namely steroids, are giving world-class athletes the boost they need to beat out their competition. But women across the globe are taking steroids every day as well, in the form of hormonal birth control. Despite their widespread use, side effects of hormonal contraceptives are largely unstudied, or have been until recently. In the last ten years, several studies have come out about the effect of taking a daily dose of steroids on women’s brains and mental health, which until now has been a severely neglected area where lack of knowledge affects millions of people worldwide.
There are millions of women taking steroids every day. But how is this possible? Are they just getting really buff? It feels like we always hear stories about how performance-enhancing drugs, namely steroids, are giving world-class athletes the boost they need to beat out their competition. But women across the globe are taking steroids every day as well, in the form of hormonal birth control. Despite their widespread use, side effects of hormonal contraceptives are largely unstudied, or have been until recently. In the last ten years, several studies have come out about the effect of taking a daily dose of steroids on women’s brains and mental health, which until now has been a severely neglected area where lack of knowledge affects millions of people worldwide. 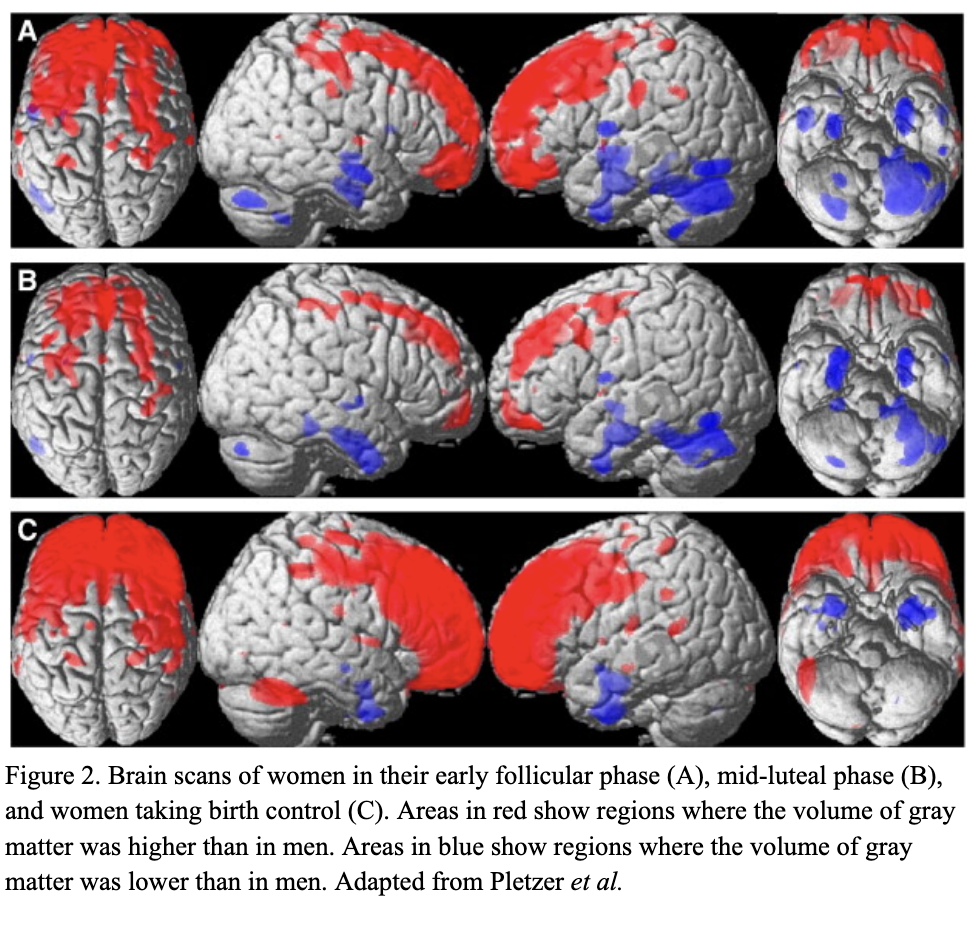 Thes
Thes