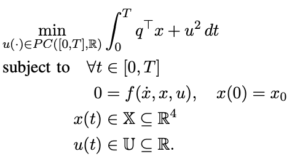Attention Deficit Hyperactivity Disorder (ADHD) is a developmental disorder that is characterized by inattention, hyperactivity, and impulsivity. This is one of the most common learning disorders diagnosed in children. ADHD not only takes a toll on an individual’s academic sphere but also their social sphere(Martin-Moratinos et al., 2023). From struggling to focus in class to having their impulsiveness affect their interpersonal relationships, symptoms permeate children’s worlds. The diagnosis process includes a variety of tests, interviews, questionnaires, and, in children, observation(ADHD Screening). For example, the Wechsler Intelligence Scale for Children-Revised (WISC-R), developed in 1974, is a test that attempts to measure intelligence through testing 10 abilities. This test, among others, such as the Wide Range Achievement Test-Revised (WRAT), was used in the diagnostic process for the participants in Abikoff et al. (1996).
In 1983, Andries Frans Sanders proposed the underaroused theory in the Cognitive-Energetic Model. The theory stated that those with ADHD have abnormally low levels of physiological arousal and, in turn, seek out input via hyperactivity(Sanders, 1983). Abikoff et al. sought to use music as a high salience extra task stimulation to investigate this theory. This study assessed the impact of music on the arithmetic performance of a group of 40 second graders with ADHD. The theorized goal was to reach an optimal level of arousal(1996). They assessed this by giving the group of 40 boys an arithmetic test to match their ability and having them complete it in different conditions. Rather than just silence or music, researchers added a speech condition. Students were subjected to three arithmetic tasks of the same difficulty. The first test was done with 10 minutes of their 3 favorite songs on loop, the second was 10 minutes of background speech, and the third and final test was 10 minutes of silence.
Through these results, researchers concluded that music was the ideal condition for kids with ADHD. This iteration of testing resulted in an accuracy of 82%, compared to 77% for speech and 79% for silence. Furthermore, when looking at the testing results of the control group, which was made up of non-disabled students at the same grade level, it was revealed that changes in condition did not affect their performance. A nuance that came to the surface through the analysis was that the effects of the conditions were order-dependent. Children who had music as their first condition had more than twice as many correct answers as those who had music as their second or third test condition.
These results lend support to the under-arousal theory. Distractibility is often a characteristic thought to be heightened in those with ADHD, and is often attributed to extra stimuli that are not related to the work they are assigned. However, Abikoff et al. offer a counter to this. Using this study as a tool, focused facilitation strategies can be developed to better support students with ADHD.
Despite the strengths of this study, it is also important to address some key limitations. It is worth glancing at the limitations of the study itself. Since this study took place in 1996, it is important to consider more recent developments in ADHD research. This could result in theories being disproved. However, even recent studies seem to support the idea that music can help with focus in ADHD patients (Martin-Moratinos et al., 2023). For example, Madjar et al showed improved reading scores in students with ADHD when exposed to music while reading(2020). Additionally, the presence of the following studies aids in the fact that the study had a relatively small sample size (40 participants), and they were all boys. Because Abikoff et al. (1996) only studied boys, it’s hard to know whether the same pattern would hold for girls with ADHD, especially since girls tend to show fewer outward hyperactive symptoms and more subtle, internalized ones. However, later work that did include girls—like Madjar et al. (2020), who tested mixed-gender preadolescents—also found that music boosted performance for students with ADHD. This suggests that the helpful effect of music isn’t limited to boys, even if the way it supports attention might look a little different across genders.
Ultimately, the management of ADHD in and out of the classroom requires an individualized, holistic approach. The demonstration of music as a coping mechanism can usher it into becoming a tool in treatment plans. In the same spirit, further development of this finding could lead to additional understandings of the impact of other types of stimuli (visual, tactile, or olfactory) on ADHD management. Overall, the study’s results open the door to using music not just as background noise, but as a strategic tool for cultivating focus in children with ADHD. As researchers expand this work—with larger, mixed-gender samples and broader types of sensory stimulation—we move closer to individualized interventions that address the whole child, both inside and outside the classroom.
References:
Abikoff, H., Courtney, M. E., Szeibel, P. J., & Koplewicz, H. S. (1996). The effects of auditory stimulation on the arithmetic performance of children with ADHD and nondisabled children. Journal of Learning Disabilities, 29(3), 238–246. https://doi.org/10.1177/002221949602900302
ADHD Screening: What To Expect. (n.d.). Cleveland Clinic. Retrieved December 24, 2025, from https://my.clevelandclinic.org/health/diagnostics/24758-adhd-screening
Everything You Need to Know About ADHD. (n.d.). Retrieved December 24, 2025, from https://www.adhdevidence.org/blog/everything-you-need-to-know-about-adhd
Madjar, N., Gazoli, R., Manor, I., & Shoval, G. (2020). Contrasting effects of music on reading comprehension in preadolescents with and without ADHD. Psychiatry Research, 291, 113207. https://doi.org/10.1016/j.psychres.2020.113207
Martin-Moratinos, M., Bella-Fernández, M., & Blasco-Fontecilla, H. (2023). Effects of Music on Attention-Deficit/Hyperactivity Disorder (ADHD) and Potential Application in Serious Video Games: Systematic Review. Journal of Medical Internet Research, 25, e37742. https://doi.org/10.2196/37742
Sanders, A. F. (1983). Towards a model of stress and human performance. Acta Psychologica, 53(1), 61–97. https://doi.org/10.1016/0001-6918(83)90016-1