Imagine you’re on a 1950s game show. The host presents three doors and lays out the rules: Behind one door is a car, and behind the other two are goats. After you choose a door, the host, knowing what’s behind each door, opens one of the remaining two doors, revealing a goat. You have the opportunity to switch. Do you?
This is, of course, the infamous Monty Hall problem. Assuming you prefer the car over the goat, the answer is to always switch, since it will give you double the probability—⅔ rather than ⅓—of winning the car. Here’s an explanation that goes through each possible case (Table 1):
Table 1: Possible outcomes for staying and switching in the Monty Hall problem (Saenen et al., 2018)

If you got it wrong, you’re not alone—between 79% and 87% of adults get it wrong, too (Saenen et al., 2018). But what is behind this phenomenon? Solving unintuitive problems like the Monty Hall problem is thought to require the inhibition of misleading information, such as from prior knowledge or false cues (Dumontheil et al., 2022; Saenen et al., 2018). However, a 2018 study by Brookman-Byrne et al. and a 2022 study by Dumontheil et al. shine a new, more nuanced light on the connection between inhibitory control and (counter)intuition.
Both studies had British schoolchildren aged 11-15 undergo a volley of tests assessing their response inhibition (the ability to manage and filter out conflicting information), semantic inhibition (the ability to suppress responses driven by impulse ), vocabulary, reasoning, and working memory. Researchers then had participants complete a set of intuitive (control) and counterintuitive math and science problems. Dumontheil et al. (2022) measured neural activity using fMRI (functional Magnetic Resonance Imaging, an imaging technique which measures blood-oxygen levels to determine which parts of the brain are active) throughout.
Unsurprisingly, researchers consistently found that participants were more accurate and faster in solving intuitive problems than counterintuitive problems. Furthermore, in counterintuitive reasoning, response inhibition predicted response times, whereas semantic inhibition predicted accuracy. Interestingly, however, the only predictors of counterintuitive reasoning ability found in both studies were a more extensive vocabulary and increased age, both of which also predicted response inhibition (Brookman-Byrne et al., 2018; Dumontheil et al., 2022). Given these unexpected findings, neuroimaging results by Dumontheil et al. (2022) were necessary to provide some insight into what goes on in participants’ brains.

Figure 1: Brain regions showing greater activation for (A) counterintuitive versus control (intuitive) problems, (C) response inhibition versus no response inhibition, and (D) semantic inhibition versus no semantic inhibition (Dumontheil et al., 2022).

Figure 2: A comparison between areas showing increased activation during counterintuitive reasoning and (A) complex inhibition behavior, and (B) interference control behavior (Dumontheil et al., 2022).
Since the overlap is limited in Figure 2, researchers concluded that the relationship between inhibitory control and counterintuitive problem solving was not direct (Dumontheil et al., 2022). They posit that the role of inhibition in counterintuitive reasoning may be limited to specific types of inhibition. In particular, semantic inhibition might be a better explanation than just response inhibition (Dumontheil et al., 2022).
Neurosynth (an fMRI image database) also associates areas activated during counterintuitive reasoning with “working memory,” “calculation,” “symbolic,” “attention,” “visually,” and “spatial,” suggesting that inhibition is not the only factor at play (Dumontheil et al., 2022). They highlight that two areas known as the intraparietal sulcus (IPS) and Brodmann area 7 (BA 7)—which together are responsible for visuo-spatial attention—show increased activation during counterintuitive reasoning, response inhibition, and semantic inhibition (Dumontheil et al., 2022). Hence, they also suggest that visuo-spatial attention may be another factor in counterintuitive reasoning (Dumontheil et al., 2022).
So what does this mean, practically? For educators, it seems that curriculum design in STEM should not be done in isolation. Given the impact of semantic reasoning, it would be prudent to balance training in purely symbolic reasoning with training in semantic reasoning (e.g., by requiring humanities classes be taken with STEM classes). For cognitive neuroscientists, this research suggests that there may be another dimension to understanding counterintuitive reasoning: the complex causal relationships between visuo-spatial attention, inhibitory control, and counterintuitive reasoning. Indeed, this is a cautionary tale about the importance of inhibition in science itself—causation is difficult to establish, and the most intuitive models in science may not always be right, either.
References
Brookman-Byrne, A., Mareschal, D., Tolmie, A. K., & Dumontheil, I. (2018, June 21). Inhibitory control and counterintuitive science and maths reasoning in adolescence. PLoS ONE, 13(6), 1-19. https://doi.org/10.1371/journal.pone.0198973
Dumontheil, I., Brookman-Byrne, A., Tolmie, A. K., & Mareschal, D. (2022). Neural and Cognitive Underpinnings of Counterintuitive Science and Math Reasoning in Adolescence. Journal of Cognitive Neuroscience, 34(7), 1205. https://doi.org/10.1162/jocn_a_01854
Saenen, L., Heyvaert, M., Van Dooren, W., Schaeken, W., & Onghena, P. (2018). Why Humans Fail in Solving the Monty Hall Dilemma: A Systematic Review. Psychologica Belgica, 58(1), 128-158. https://doi.org/10.5334/pb.274







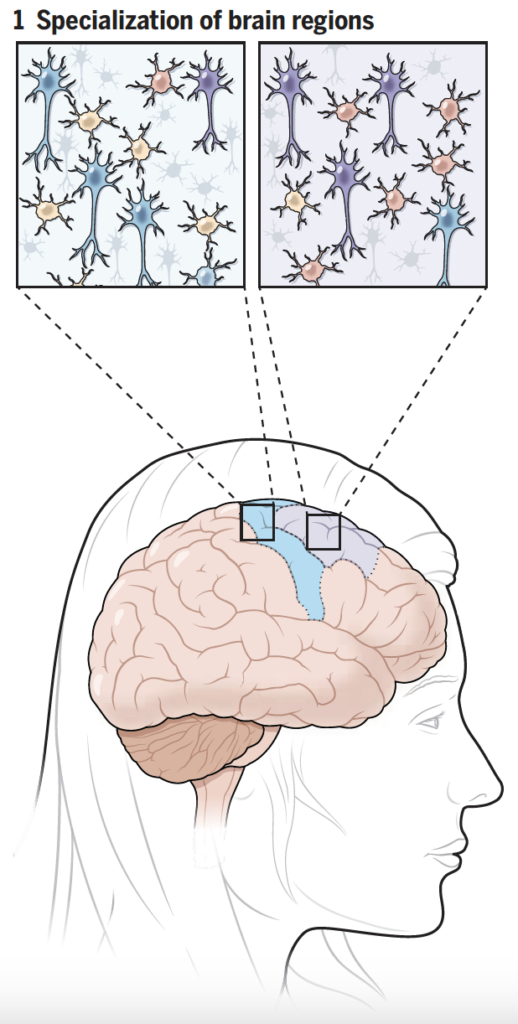
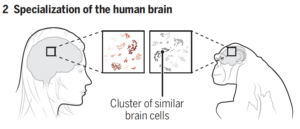
 Comparisons of cell composition in regions across the brain resulted in findings from researchers under Siletti from the University of North Carolina at Chapel Hill and Jorstad from Harvard University. The two groups found that rather than mainly having different types of cells in different parts of the brain, some different parts of the brain shared the same cells but had different proportions of these cells. There were some exceptions, such as inhibitory neurons in the primary visual cortex, although the explanation of this finding is unclear. Such results change the understanding of evolutionary diversity in that diversification does not depend heavily on having many different cell types, but rather on having varying proportions of cells with small differences.
Comparisons of cell composition in regions across the brain resulted in findings from researchers under Siletti from the University of North Carolina at Chapel Hill and Jorstad from Harvard University. The two groups found that rather than mainly having different types of cells in different parts of the brain, some different parts of the brain shared the same cells but had different proportions of these cells. There were some exceptions, such as inhibitory neurons in the primary visual cortex, although the explanation of this finding is unclear. Such results change the understanding of evolutionary diversity in that diversification does not depend heavily on having many different cell types, but rather on having varying proportions of cells with small differences.